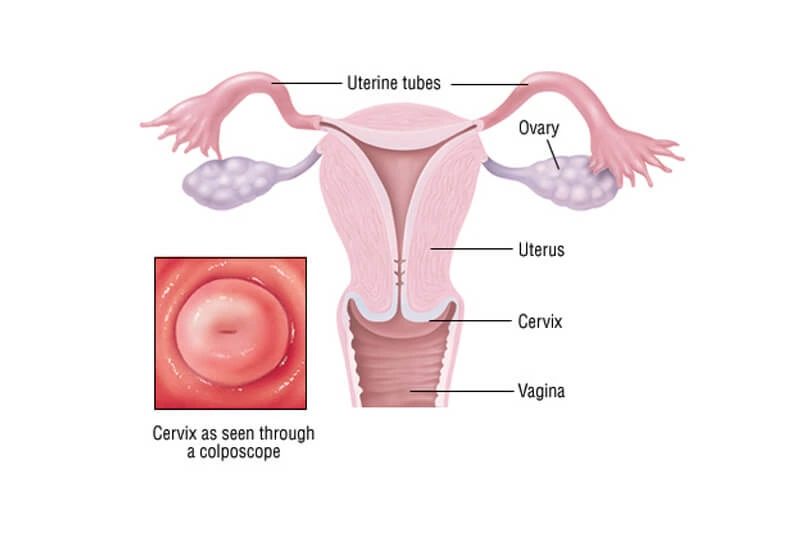
Cervical cancer starts in the cells of the cervix—the lower, narrow part of the uterus that connects to the vagina.
Most cases are linked to infection with certain types of human papillomavirus (HPV), a sexually transmitted virus.
In many people the immune system clears HPV naturally. In some, persistent infection can lead to changes in cervical cells that may develop into cancer over time.
Screening and vaccination greatly reduce the risk of cervical cancer by detecting cell changes early and preventing high-risk HPV infection.
The endocervix (closest to the uterus) is lined with glandular cells.
The ectocervix (next to the vagina) is covered in squamous cells.
These cell types meet at the transformation zone. Its location shifts with age and after childbirth — and it’s where most abnormal cell changes begin.

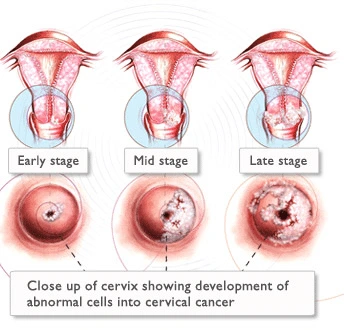

Cervical cancers and cervical pre-cancers are classified by how they look under a microscope. The main types of cervical cancers are squamous cell carcinoma and adenocarcinoma.
Cryotherapy is extensively used to treat ectocervical premalignant lesions that occupy less than 75% of cervix and can be covered by the cryo-probe. For low- and middle-income countries (LMIC) the World Health Organization (WHO) recommends that cryotherapy be used to treat screen positive women (HPV Test or Visual Inspection with Acetic Acid (VIA)), even without colposcopic or histologic verification of disease.
This ‘single visit approach’ will significantly increase the demand for cryotherapy in these countries. Unfortunately, cryotherapy has certain logistic disadvantages that can limit its use, such as procuring refrigerant gas and ensuring its regular supply. In many LMICs, transporting heavy gas tanks is inconvenient and sometimes impossible in remote areas.
For this reason, it is essential to find an alternative to cryotherapy that is effective, safe, suitable and sustainable for LMICs.

Thermal coagulation™, using a portable thermocoagulator™, destroys the transformation zone at temperatures in the 100°C to 120°C range. It is an outpatient procedure, easily learned and can be performed by a range of providers including nurses.
Because there are very few recent reports of its safety and effectiveness in the published literature, and because cryotherapy predominated the practice, WHO has not included thermal ablation in its recently updated recommendations.

Because there are very few recent reports of its safety and effectiveness in the published literature, and because cryotherapy predominated the practice, WHO has not included thermal ablation in its recently updated recommendations.
Liger Medical has developed a novel hand thermal coagulator for the treatment of cervical pre-cancers (tc thermocoagulator™), which has design advantages over the Semm’s™ cold coagulation. Briefly, it is a hand-held, lightweight, inexpensive, battery operated, cordless device, which can perform approximately 50 procedures with a single fully charged battery. It is likely to be suitable for programs of cervical cancer prevention in LMICs as running electricity is not required.
In vitro studies by the manufacturers suggest that it reaches the needed temperature quicker than the Semm™ coagulation (10 seconds), maintains the pre-set temperature more consistently than the Semm™ model, whilst at the same time it achieves a similar depth of destruction and does not produce smoke nor stick to the tissue post-treatment.
1. WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. Geneva: World Health Organization; 2013.
2. Duncan ID. The Semm™ cold coagulation in the management of cervical intraepithelial neoplasia. Clin Obstet Gynecol 1983 Dec;26(4):996–1006.
3. Dolman L, Sauvaget C, Muwonge R, Sankaranarayanan R. Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia: a systematic review. BJOG 2014 Jul;121(8):929–42.
4. de Sanjosé et al. eLife 2023;12:RP91469. DOI: https://doi.org/10.7554/eLife.91469
5. JNCI: Journal of the National Cancer Institute, 2025, 00(0), 1–6 https://doi.org/10.1093/jnci/djaf054 Advance Access Publication Date: March 18, 2025